Which of the Following Measurements Contains Two Significant Figures
2 0 3 m. Given P00030 m Q240 m R3000 m Significant figures in PQR are respectively.
Chemistry Lesson Significant Digits Measurements Get Chemistry Help
The number of significant figures in this mass is - What is the mass of carbon in dimethylsulfoxide c2h6so rounded to three significant figures.
. Express the product of 22 mm and 500 mm using the correct number of significant digits. If you use this calculator for the calculation and you enter only 2 for the radius value the calculator will read the 2 as one significant figure. 2 235 470.
Express the answer in significant figures. Compute the area given the following measurements. A 500 b 50 102 c 395 d 100 103 e 0001 f 000100 2 Round each number to three significant figures.
055 has only two significant figures so the answer can have only two significant. If an average of 4 trials is being calculated the 4 is infinitely significant. Figs 45 2 sig.
Area 21 cm x 324 cm 6804 cm2 We note that 21 contains two significant figures while 324 contains three significant figures. A measurement contains significant figures and units. The number 306 means that the true value rests somewhere between 305 and 307 thus the zero is known with certainty and is significant.
Count how many significant figures are in a number and find which digits are significant. All digits of a measured quantity including the certain one are called significant figures. 1 Which of the following measurements contain three significant figures.
Counting Significant Figures Examples 1. Each side of a cube is measured to be 7. Express the sum of 768 m and 50 m using the correct number of significant digits.
Significant Figures Scientific measurements are reported so that every digit is certain except the last which is estimated. The most precise measurement. The correct answer is C the measurement that contains two significant figure is 00000 44 L.
Which of the following measurements contains 2 significant figures. All numbers should be recorded from a digital readout. Test your ability to find how many significant figures are in a number.
883 cm² When multiplying or dividing measurements does the answer have the same amount of significant figures as the measurement with the most significant figures. The uncertain digit is estimated between the last two markings. 1307g 13071 g 13g 131 g 10 g How many significant figures should be reported in the answer to the following calculation.
The trailing zeros present at the right side of the decimal point are significant figures. 000033 contains two significant figures. 0408g 9040 K 00400ms 905 times 1024 m The correct answer for the addition of 75 g 226 g 1311g 2g is _____.
Enter whole numbers real numbers scientific notation or e notation. All non-zero digits are always significant. You can use this calculator for significant figures practice.
When multiplying and dividing measured quantities the number of significant figures in the result should be equal to the number of significant figures in __________. Which of the following measurements contains two significant figures. Trailing zeros of the number without a decimal point are not s ignificant figures.
How many significant figures should be retained in the result of the following calculation. Which of the following measurements contains two significant figures. A compound has a mass of 26632 102 gmol.
All of the measurements. The trailing zeros present in the whole number with the decimal point are significant figures. Which of the following measurements contains two significant figures.
If you measure a radius of 235 multiply by 2 to find the diameter of the circle. Read the length of the metal bar with the correct number of significant figures. The chief advantage of the metric system over other systems of measurement is that it ____.
20546 cm and 43 cm. RULE 2 - Zero is significant when it is between two non-zero digits The quantities 306 306 306 and 0306 all contain 3 significant figures since the 0 between the 3 and 6 is significant. Therefore our answer would be recorded as 68 cm2.
All other options provided are not correct because they have three significant figures. 00004 00 L has three significant figures 00004 04 L has three significant figures 00004 40 L has three significant figures. Example inputs are 3500 350056 35 x 103 and 35e3.
Our product should contain no more than two significant figures. A 00240 С 00901 B 371 D 1010 E 0004. A 0036549 b 1044987 c 40007 3 Label each of the following digits as significant or not significant.
The least and most precise measurement. When multiplying or dividing the answer is rounded to the fewest significant figures in the given measurements. How many significant figures does the following number have.
Chemistry Lesson Significant Digits Measurements Get Chemistry Help
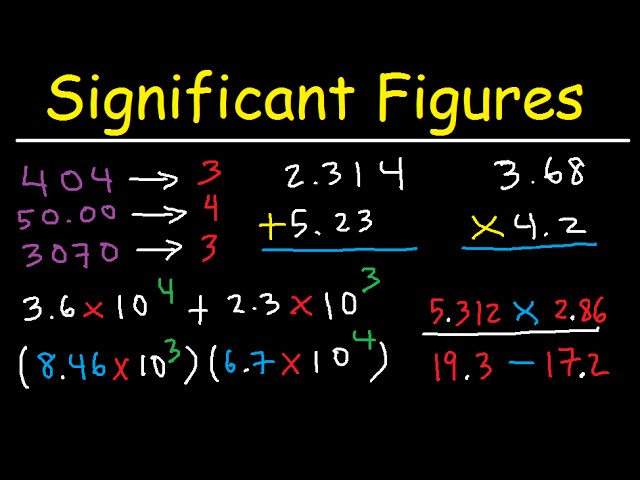
Significant Figures Made Easy Youtube
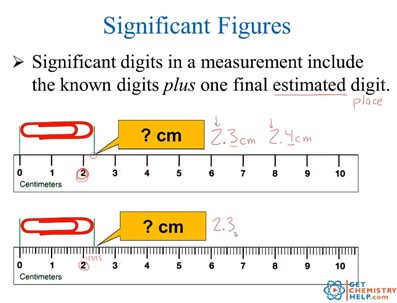
Chemistry Lesson Significant Digits Measurements Get Chemistry Help
Comments
Post a Comment